ACT Science Practice Test 1
Exam Summary
0 of 14 Questions completed
Questions:
Information
You have already completed the exam before. Hence you can not start it again.
Exam is loading…
You must sign in or sign up to start the exam.
You must first complete the following:
Results
Results
0 of 14 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
-

Need More Help?
Read our reviews of the best ACT prep courses. We reviewed the best providers and have exclusive discounts you can use.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 14
1. Question
Data Representation (1 of 7)
Exercising elicits an acute hormonal response. The magnitude of this response is dependent on the mode and intensity of exercise. Figure 1 shows the concentration of two hormones in response to exercise as measured by researchers in pmol/l and nmol/l (1 pmol/l = .001 nmol/l). Measurements were taken at multiple timestamps before beginning the workout, after the completion of each exercise in the workout, 15 minutes after completing the workout, and 30 minutes after completing the workout. Changes in these hormones were tracked across two different exercise conditions, or modes, defined as MR and FR.
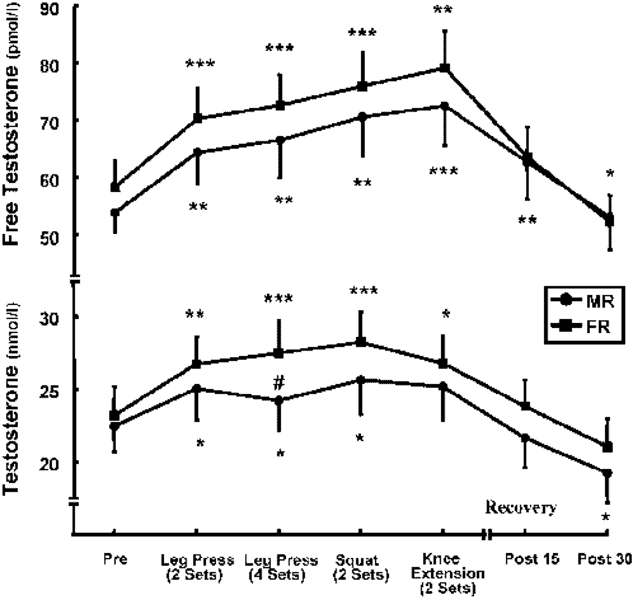
Figure adapted from Acute hormonal and neuromuscular responses and recovery to forced vs. Maximum repetitions multiple resistance exercises by Ahtianinen et al.
In both the MR and FR conditions, Free Testosterone concentration exhibits a trend during the duration of the workout. What is that trend?
CorrectIncorrect -
Question 2 of 14
2. Question
Data Representation (2 of 7)
Exercising elicits an acute hormonal response. The magnitude of this response is dependent on the mode and intensity of exercise. Figure 1 shows the concentration of two hormones in response to exercise as measured by researchers in pmol/l and nmol/l (1 pmol/l = .001 nmol/l). Measurements were taken at multiple timestamps before beginning the workout, after the completion of each exercise in the workout, 15 minutes after completing the workout, and 30 minutes after completing the workout. Changes in these hormones were tracked across two different exercise conditions, or modes, defined as MR and FR.

Figure adapted from Acute hormonal and neuromuscular responses and recovery to forced vs. Maximum repetitions multiple resistance exercises by Ahtianinen et al.
In both the MR and FR conditions, Testosterone concentration is implied to peak by a certain time stamp. What is that timestamp?
CorrectIncorrect -
Question 3 of 14
3. Question
Data Representation (3 of 7)
Exercising elicits an acute hormonal response. The magnitude of this response is dependent on the mode and intensity of exercise. Figure 1 shows the concentration of two hormones in response to exercise as measured by researchers in pmol/l and nmol/l (1 pmol/l = .001 nmol/l). Measurements were taken at multiple timestamps before beginning the workout, after the completion of each exercise in the workout, 15 minutes after completing the workout, and 30 minutes after completing the workout. Changes in these hormones were tracked across two different exercise conditions, or modes, defined as MR and FR.

Figure adapted from Acute hormonal and neuromuscular responses and recovery to forced vs. Maximum repetitions multiple resistance exercises by Ahtianinen et al.
At the Pre timestamp, is there a higher concentration of Free Testosterone in the FR condition or Testosterone in the MR condition?
CorrectIncorrect -
Question 4 of 14
4. Question
Data Representation (4 of 7)
Exercising elicits an acute hormonal response. The magnitude of this response is dependent on the mode and intensity of exercise. Figure 1 shows the concentration of two hormones in response to exercise as measured by researchers in pmol/l and nmol/l (1 pmol/l = .001 nmol/l). Measurements were taken at multiple timestamps before beginning the workout, after the completion of each exercise in the workout, 15 minutes after completing the workout, and 30 minutes after completing the workout. Changes in these hormones were tracked across two different exercise conditions, or modes, defined as MR and FR.

Figure adapted from Acute hormonal and neuromuscular responses and recovery to forced vs. Maximum repetitions multiple resistance exercises by Ahtianinen et al.
At which timestamp is there the greatest difference in Testosterone concentration between the MR and FR conditions?
CorrectIncorrect -
Question 5 of 14
5. Question
Data Representation (5 of 7)
Exercising elicits an acute hormonal response. The magnitude of this response is dependent on the mode and intensity of exercise. Figure 1 shows the concentration of two hormones in response to exercise as measured by researchers in pmol/l and nmol/l (1 pmol/l = .001 nmol/l). Measurements were taken at multiple timestamps before beginning the workout, after the completion of each exercise in the workout, 15 minutes after completing the workout, and 30 minutes after completing the workout. Changes in these hormones were tracked across two different exercise conditions, or modes, defined as MR and FR.

Figure adapted from Acute hormonal and neuromuscular responses and recovery to forced vs. Maximum repetitions multiple resistance exercises by Ahtianinen et al.
Suppose the researchers added another exercise to the end of the workout in the FR condition and measured Free Testosterone concentration at that point. If the current trend continued, what would be the most likely concentration observed at that timestamp?
CorrectIncorrect -
Question 6 of 14
6. Question
Data Representation (6 of 7)
Exercising elicits an acute hormonal response. The magnitude of this response is dependent on the mode and intensity of exercise. Figure 1 shows the concentration of two hormones in response to exercise as measured by researchers in pmol/l and nmol/l (1 pmol/l = .001 nmol/l). Measurements were taken at multiple timestamps before beginning the workout, after the completion of each exercise in the workout, 15 minutes after completing the workout, and 30 minutes after completing the workout. Changes in these hormones were tracked across two different exercise conditions, or modes, defined as MR and FR.

Figure adapted from Acute hormonal and neuromuscular responses and recovery to forced vs. Maximum repetitions multiple resistance exercises by Ahtianinen et al.
In both the MR and FR conditions for Testosterone, how do concentrations at the Post 30 timestamp relate to concentrations at the Pre timestamp?
CorrectIncorrect -
Question 7 of 14
7. Question
Data Representation (7 of 7)
Exercising elicits an acute hormonal response. The magnitude of this response is dependent on the mode and intensity of exercise. Figure 1 shows the concentration of two hormones in response to exercise as measured by researchers in pmol/l and nmol/l (1 pmol/l = .001 nmol/l). Measurements were taken at multiple timestamps before beginning the workout, after the completion of each exercise in the workout, 15 minutes after completing the workout, and 30 minutes after completing the workout. Changes in these hormones were tracked across two different exercise conditions, or modes, defined as MR and FR.

Figure adapted from Acute hormonal and neuromuscular responses and recovery to forced vs. Maximum repetitions multiple resistance exercises by Ahtianinen et al.
In general, is Testosterone concentration greater in the FR or MR condition?
CorrectIncorrect -
Question 8 of 14
8. Question
Research Summary (1 of 7)
Use this passage for the next 7 questions:
A chemist performed two experiments. The chemist had the objective of determining the melting points and boiling points of multiple elements. When an element reaches its Melting Point or Boiling Point, its state of matter changes. A solid object heated to its Melting Point becomes a liquid, and if further heated to its Boiling Point it becomes a gas. If a gas is cooled to its Boiling Point it will transition back to a liquid, and if cooled further to its Melting Point it will transition back to a solid.
In Experiment 1, the chemist gathered eight elements and stored them each individually at temperatures that allowed them to exist in their solid state. Following this, the chemist heated each element and recorded the temperature at which melting occurred. The results are shown in Figure 1.
Element Melting Point (Celsius) Hydrogen -259.16 Magnesium 650 Argon -189.34 Oxygen -218.79 Phosphorus 44.15 Lithium 180.5 Sodium 97.79 Barium 727 Figure 1
In Experiment 2, the chemist stored each of the eight elements used in Experiment 1 at temperatures that allowed them to exist in their liquid state. Following this, the chemist heated each element and recorded the temperature at which boiling occurred. The results are shown in Figure 2.
Element Boiling Point (Celsius) Hydrogen -252.87 Magnesium 1090 Argon -185.85 Oxygen -182.96 Phosphorus 280.5 Lithium 1342 Sodium 882.94 Barium 1845 Figure 2
Based on the results of Experiments 1 and 2, it is safe to conclude:
CorrectIncorrect -
Question 9 of 14
9. Question
Research Summary (2 of 7)
A chemist performed two experiments. The chemist had the objective of determining the melting points and boiling points of multiple elements. When an element reaches its Melting Point or Boiling Point, its state of matter changes. A solid object heated to its Melting Point becomes a liquid, and if further heated to its Boiling Point it becomes a gas. If a gas is cooled to its Boiling Point it will transition back to a liquid, and if cooled further to its Melting Point it will transition back to a solid.
In Experiment 1, the chemist gathered eight elements and stored them each individually at temperatures that allowed them to exist in their solid state. Following this, the chemist heated each element and recorded the temperature at which melting occurred. The results are shown in Figure 1.
Element Melting Point (Celsius) Hydrogen -259.16 Magnesium 650 Argon -189.34 Oxygen -218.79 Phosphorus 44.15 Lithium 180.5 Sodium 97.79 Barium 727 Figure 1
In Experiment 2, the chemist stored each of the eight elements used in Experiment 1 at temperatures that allowed them to exist in their liquid state. Following this, the chemist heated each element and recorded the temperature at which boiling occurred. The results are shown in Figure 2.
Element Boiling Point (Celsius) Hydrogen -252.87 Magnesium 1090 Argon -185.85 Oxygen -182.96 Phosphorus 280.5 Lithium 1342 Sodium 882.94 Barium 1845 Figure 2
If the chemist had continued heating the elements in Experiment 1 past their Melting Point until they boiled without stopping, would the results be the same as in Experiment 2?
CorrectIncorrect -
Question 10 of 14
10. Question
Research Summary (3 of 7)
A chemist performed two experiments. The chemist had the objective of determining the melting points and boiling points of multiple elements. When an element reaches its Melting Point or Boiling Point, its state of matter changes. A solid object heated to its Melting Point becomes a liquid, and if further heated to its Boiling Point it becomes a gas. If a gas is cooled to its Boiling Point it will transition back to a liquid, and if cooled further to its Melting Point it will transition back to a solid.
In Experiment 1, the chemist gathered eight elements and stored them each individually at temperatures that allowed them to exist in their solid state. Following this, the chemist heated each element and recorded the temperature at which melting occurred. The results are shown in Figure 1.
Element Melting Point (Celsius) Hydrogen -259.16 Magnesium 650 Argon -189.34 Oxygen -218.79 Phosphorus 44.15 Lithium 180.5 Sodium 97.79 Barium 727 Figure 1
In Experiment 2, the chemist stored each of the eight elements used in Experiment 1 at temperatures that allowed them to exist in their liquid state. Following this, the chemist heated each element and recorded the temperature at which boiling occurred. The results are shown in Figure 2.
Element Boiling Point (Celsius) Hydrogen -252.87 Magnesium 1090 Argon -185.85 Oxygen -182.96 Phosphorus 280.5 Lithium 1342 Sodium 882.94 Barium 1845 Figure 2
Suppose that after the completion of Experiment 2, the chemist wanted to test the state of matter each element would be in at room temperature (23 degrees Celsius). How would the chemist most effectively do this?
CorrectIncorrect -
Question 11 of 14
11. Question
Research Summary (4 of 7)
A chemist performed two experiments. The chemist had the objective of determining the melting points and boiling points of multiple elements. When an element reaches its Melting Point or Boiling Point, its state of matter changes. A solid object heated to its Melting Point becomes a liquid, and if further heated to its Boiling Point it becomes a gas. If a gas is cooled to its Boiling Point it will transition back to a liquid, and if cooled further to its Melting Point it will transition back to a solid.
In Experiment 1, the chemist gathered eight elements and stored them each individually at temperatures that allowed them to exist in their solid state. Following this, the chemist heated each element and recorded the temperature at which melting occurred. The results are shown in Figure 1.
Element Melting Point (Celsius) Hydrogen -259.16 Magnesium 650 Argon -189.34 Oxygen -218.79 Phosphorus 44.15 Lithium 180.5 Sodium 97.79 Barium 727 Figure 1
In Experiment 2, the chemist stored each of the eight elements used in Experiment 1 at temperatures that allowed them to exist in their liquid state. Following this, the chemist heated each element and recorded the temperature at which boiling occurred. The results are shown in Figure 2.
Element Boiling Point (Celsius) Hydrogen -252.87 Magnesium 1090 Argon -185.85 Oxygen -182.96 Phosphorus 280.5 Lithium 1342 Sodium 882.94 Barium 1845 Figure 2
If each element were returned to its state at room temperature (23 degrees Celsius), how many elements would be solid?
CorrectIncorrect -
Question 12 of 14
12. Question
Research Summary (5 of 7)
A chemist performed two experiments. The chemist had the objective of determining the melting points and boiling points of multiple elements. When an element reaches its Melting Point or Boiling Point, its state of matter changes. A solid object heated to its Melting Point becomes a liquid, and if further heated to its Boiling Point it becomes a gas. If a gas is cooled to its Boiling Point it will transition back to a liquid, and if cooled further to its Melting Point it will transition back to a solid.
In Experiment 1, the chemist gathered eight elements and stored them each individually at temperatures that allowed them to exist in their solid state. Following this, the chemist heated each element and recorded the temperature at which melting occurred. The results are shown in Figure 1.
Element Melting Point (Celsius) Hydrogen -259.16 Magnesium 650 Argon -189.34 Oxygen -218.79 Phosphorus 44.15 Lithium 180.5 Sodium 97.79 Barium 727 Figure 1
In Experiment 2, the chemist stored each of the eight elements used in Experiment 1 at temperatures that allowed them to exist in their liquid state. Following this, the chemist heated each element and recorded the temperature at which boiling occurred. The results are shown in Figure 2.
Element Boiling Point (Celsius) Hydrogen -252.87 Magnesium 1090 Argon -185.85 Oxygen -182.96 Phosphorus 280.5 Lithium 1342 Sodium 882.94 Barium 1845 Figure 2
If each element were returned to its state at room temperature (23 degrees Celsius), how many elements would be liquid?
CorrectIncorrect -
Question 13 of 14
13. Question
Research Summary (6 of 7)
A chemist performed two experiments. The chemist had the objective of determining the melting points and boiling points of multiple elements. When an element reaches its Melting Point or Boiling Point, its state of matter changes. A solid object heated to its Melting Point becomes a liquid, and if further heated to its Boiling Point it becomes a gas. If a gas is cooled to its Boiling Point it will transition back to a liquid, and if cooled further to its Melting Point it will transition back to a solid.
In Experiment 1, the chemist gathered eight elements and stored them each individually at temperatures that allowed them to exist in their solid state. Following this, the chemist heated each element and recorded the temperature at which melting occurred. The results are shown in Figure 1.
Element Melting Point (Celsius) Hydrogen -259.16 Magnesium 650 Argon -189.34 Oxygen -218.79 Phosphorus 44.15 Lithium 180.5 Sodium 97.79 Barium 727 Figure 1
In Experiment 2, the chemist stored each of the eight elements used in Experiment 1 at temperatures that allowed them to exist in their liquid state. Following this, the chemist heated each element and recorded the temperature at which boiling occurred. The results are shown in Figure 2.
Element Boiling Point (Celsius) Hydrogen -252.87 Magnesium 1090 Argon -185.85 Oxygen -182.96 Phosphorus 280.5 Lithium 1342 Sodium 882.94 Barium 1845 Figure 2
If the Melting Point and Boiling Point characteristics of experiments 1 and 2 were to be generalized to all other elements, which of these statements would be false?
CorrectIncorrect -
Question 14 of 14
14. Question
Research Summary (7 of 7)
A chemist performed two experiments. The chemist had the objective of determining the melting points and boiling points of multiple elements. When an element reaches its Melting Point or Boiling Point, its state of matter changes. A solid object heated to its Melting Point becomes a liquid, and if further heated to its Boiling Point it becomes a gas. If a gas is cooled to its Boiling Point it will transition back to a liquid, and if cooled further to its Melting Point it will transition back to a solid.
In Experiment 1, the chemist gathered eight elements and stored them each individually at temperatures that allowed them to exist in their solid state. Following this, the chemist heated each element and recorded the temperature at which melting occurred. The results are shown in Figure 1.
Element Melting Point (Celsius) Hydrogen -259.16 Magnesium 650 Argon -189.34 Oxygen -218.79 Phosphorus 44.15 Lithium 180.5 Sodium 97.79 Barium 727 Figure 1
In Experiment 2, the chemist stored each of the eight elements used in Experiment 1 at temperatures that allowed them to exist in their liquid state. Following this, the chemist heated each element and recorded the temperature at which boiling occurred. The results are shown in Figure 2.
Element Boiling Point (Celsius) Hydrogen -252.87 Magnesium 1090 Argon -185.85 Oxygen -182.96 Phosphorus 280.5 Lithium 1342 Sodium 882.94 Barium 1845 Figure 2
Suppose that after the experiment the chemist made a mistake and mixed up storage of the elements Oxygen and Argon. How would the chemist be able to identify which element is which if both are currently at a room temperature of 23 degrees Celsius?
CorrectIncorrect
